Hi All,
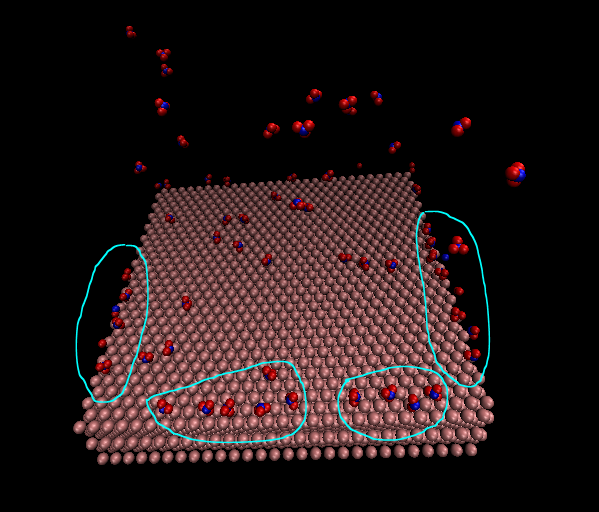
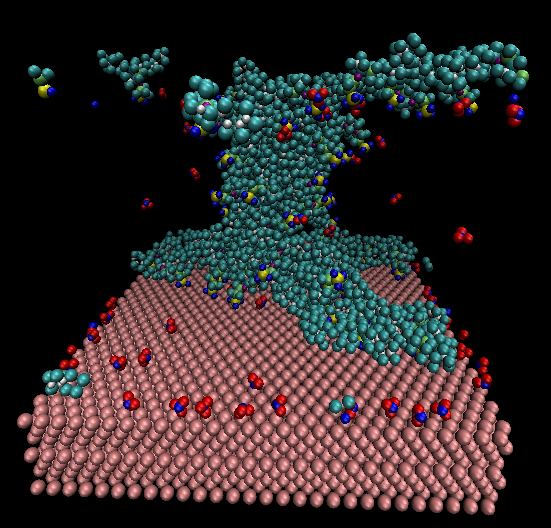
I am modelling a polymer system in the water on Pt (111) surface. The polymer has several side chains that are terminated with an acid group (SO3-). For making the system neutral, I adding hydronium molecules in the system. The applied boundary condition is periodic for x and y direction and fixed for the z-axis. The long-ranged coulombic interaction has been used (pppm approach). As you can see in the Pics, the hydronium molecules mostly are on the boundary, which I do not know why. Do you have any suggestions for it? Do you think that the boundaries have a problem?
Thanks,
Atefeh
Hi All,
I am modelling a polymer system in the water on Pt (111) surface. The polymer has several side chains that are terminated with an acid group (SO3-). For making the system neutral, I adding hydronium molecules in the system. The applied boundary condition is periodic for x and y direction and fixed for the z-axis. The long-ranged coulombic interaction has been used (pppm approach). As you can see in the Pics, the hydronium molecules mostly are on the boundary, which I do not know why. Do you have any suggestions for it? Do you think that the boundaries have a problem?
it is impossible to infer a “trend” from just a single observation. also your system seem very small to assume that atoms would move unaffected by other components in the system.
if you add charged particles to a system of (partial) charges, they will go to locations based on their kinetic energy and the electrostatic potential.
of course, each added particle will change the potential.
axel.

