Title: I need some clarifications on the Coulomb interaction in Lammps.
For pair potentials such as Born?CMayer?CHuggins, there is an adding Coulombic pairwise interaction which is expressed as: (Page 1103 in the latest Manual).
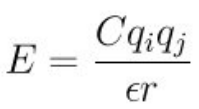
C is an energy-conversion constant. However, in some references(Masahiko Matsumiya, Ryuzo Takagi, Electrochimica Acta 46 (2001) 3563?C3572), the Coulombic interaction is: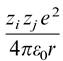
Otherwise, some other references (Jamshed Anwar et al, J. Chem. Phys., Vol. 118, No. 2, 8 January 2003) formulate the Coulombic interaction as: 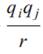 .
.
My questions:
-
What is the energy-conversion constant means? What is the value and unit of it? Does it have any relationships with the electrostatic constant?
-
Why the energy-conversion constant is ignored sometimes? Should I modify the energy-conversion constant to satisfy the references’ expressions? If so, how to modify the energy-conversion constant.
-
Does the charge numbers are always integers? E.g. the charge of Na+ in NaCl is +1 with enough precision?
Thank you very much!
Title: I need some clarifications on the Coulomb interaction in Lammps.
For pair potentials such as Born–Mayer–Huggins, there is an adding
Coulombic pairwise interaction which is expressed as: (Page 1103 in the
latest Manual).
C is an energy-conversion constant. However, in some references(Masahiko
Matsumiya, Ryuzo Takagi, Electrochimica Acta 46 (2001) 3563–3572), the
Coulombic interaction is:
Otherwise, some other references (Jamshed Anwar et al, J. Chem. Phys.,
Vol. 118, No. 2, 8 January 2003) formulate the Coulombic interaction as: .
My questions:
1. What is the energy-conversion constant means? What is the value
and unit of it? Does it have any relationships with the electrostatic
constant?
it is essentially a unit conversion factor. its value depends on what unit
of energy and what units of charge you have.
2. Why the energy-conversion constant is ignored sometimes? Should I
modify the energy-conversion constant to satisfy the references’
expressions? If so, how to modify the energy-conversion constant.
see answer to question 1.
3. Does the charge numbers are always integers? E.g. the charge of
Na+ in NaCl is +1 with enough precision?
no. that is model dependent, but for salts it is often chosen to be
integers.
axel.
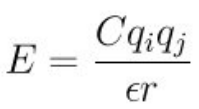
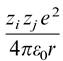
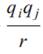
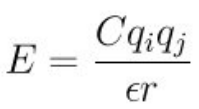
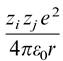
![]() .
.