Dear all,
I am new in LAMMPS and I have to calculate the volumetric thermal expansion coefficient (AlphaP) by performing on-the-fly Analysis.
To do this, I created input script below, where the AlphaP is calculated by using equation (10) of the following articles:
https://pubs.acs.org/doi/10.1021/ct200731v
The equation (10) is <δVδH>=kBT^2**[AlphaP],
where V, H, kB, and T are volume, enthalpy, Boltzmann constant, and temperature, respectively. Here, δV and δH mean V- and H-; the fluctuations of volume and enthalpy.
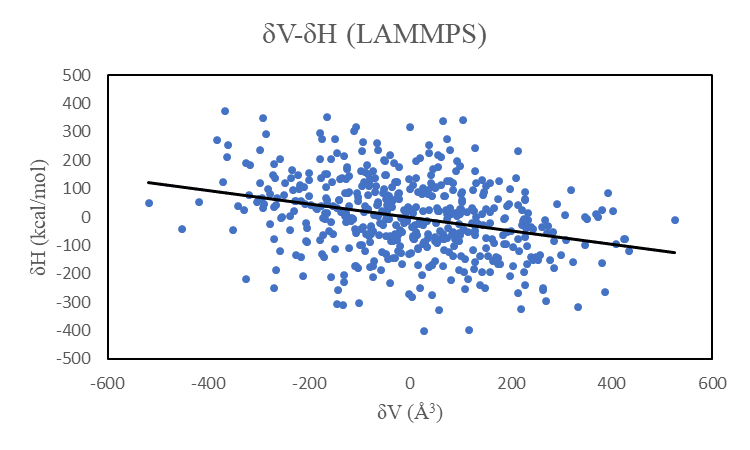
I tried this analysis towards 500 water molecules at NPT ensemble during 2ns simulations after equilibrium of initial 2.552 nm^3 box.
However, I gained negative values of AlphaP (-0.0027/K) at 300K, because δV and δH have a negative correlation coefficient (-0.30).
(The version is LAMMPS 64-bit 5Sep2018.)
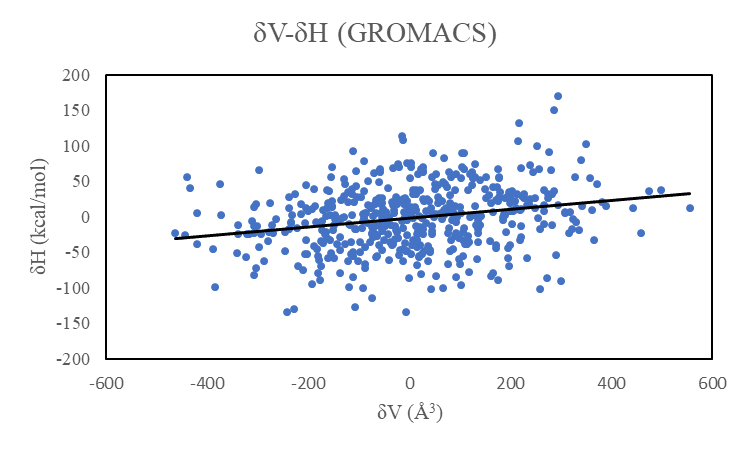
When I tried towards the same system in GROMACS, AlphaP is +0.00060/K and the correlation coefficient between δV and δH is +0.24.
(the experiment data of AlphaP is +0.000256/K.)
I have attached the correlation graphs of δV-δH in LAMMPS and GROMACS. The δH in LAMMPS is larger than that in GROMACS.
This is my input file in LAMMPS.