Hi all
I intend to calculate heat capacity at constant pressure (at 1 atmosphere) of water(TIP3P model).
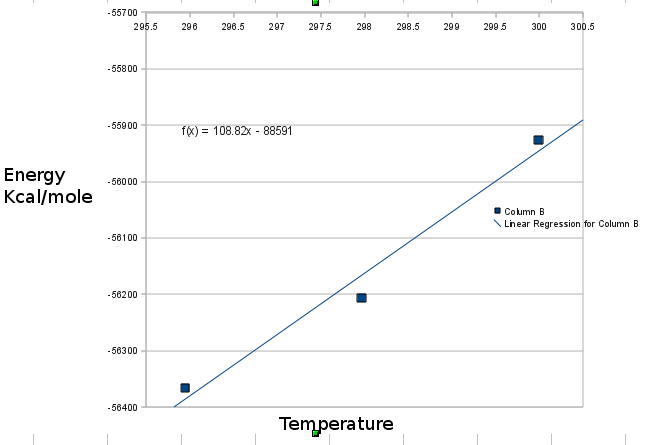
For this propose, first I have calculated total energy for three point of temperature 296,298,300 K,
Then ploted total energy against temperature and calculated slope of it. But, it is gotten 108 in real unit (Kcal/mole). While it must be 1 Kcal/mole.
I used,once, SHAKE algorithm and next without SHAKE algorithm. system contains 7000 water molecules.
the system equilibrated in NVE + langevin for 150ps then turn off them and then turn on npt for 300 ps.
Please help me to solve this.
Thanks in advance
Farrokh
Hi all
I intend to calculate heat capacity at constant pressure (at 1 atmosphere)
of water(TIP3P model).
For this propose, first I have calculated total energy for three point of
temperature 296,298,300 K,
Then ploted total energy against temperature and calculated slope of it.
But, it is gotten 108 in real unit (Kcal/mole). While it must be 1
Kcal/mole.
do you have any literature confirming that this is a suitable way
to compute the specific heat?
i always thought that you need to do path-integral MD with a large
number of PI-beads to get half-way decent results.
check out, e.g., Phys. Rev. E 71 041204 (2005)
I used,once, SHAKE algorithm and next without SHAKE algorithm. system
TIP3P is a rigid water potential and thus requires using SHAKE.
axel.
Don’t forget the temperature K in the unit (Kcal/mole/K). I doubt your “1 Kcal/mole”. You should go back to check it because it is a little tricky in unit conversion. The water at 25 centidegree has **4.1813 J/g/K (**75.327 J/mol/K).
Another thing is that, as Alex mentioned, please make sure you did it the right way. I recommend you read the Chapter 2.5 in the book “Computer simulation of liquids” by M.P. Allen. It has the details to calculate the specific heat/heat capacity.
Good luck and Happy New Year!
Xiaopeng
