Dear all, I use LAMMPS to simulate polymer in salt solution, and the force field is PCFF. However, I found that in my salt solution, sodium ions, chloride ions and calcium ions gradually gathered to form a structure similar to precipitation, which is not consistent with the expected situation. Is there any similar situation, or is the PCFF force field not applicable to salt solution? Force field documents have been attached.
pcff_interface.frc (386 KB)
Test1.data (182.6 KB)
Test1.in (1.6 KB)
I would suspect the latter, but the question you should ask (yourself) is: is there any publication where people have successfully done simulations of PCFF in electrolytes?
Also, have you computed the concentration of you ions? Perhaps your concentration is simply too high.
One alarm bell ringing is the mention of calcium ion. Divalent cations are particularly difficult to model with non-polarizable classical force fields. Their “effective” charge is usually lower than 2+ and there is charge density exchange with neighboring atoms, which cannot be represented with a non-polarizable classical model.
I tried other models with lower concentration, and the results were similar. It should not be the concentration. I think maybe the force field is not applicable.
That does not answer my question. How low a concentration have tried?
I tried the solution of 0.2m CaCl2 and 0.2mnacl. At this time, the calcium ion is not complexed with the sodium ion, but the calcium ion still keeps a close distance with the chloride ion, as shown in the figure, Na+, purple, Ca2+, cyan, Cl-, green, water molecules not shown
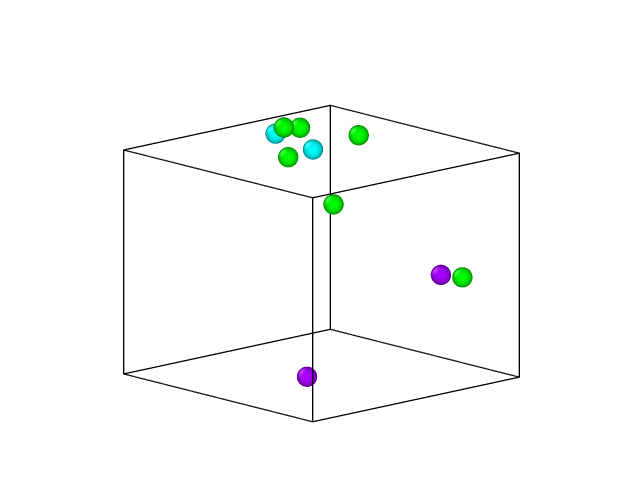
That is still pretty “salty”. If my math is correct, the maximum solubility of CaCl2 in water is about 2/3rd mol/l. However, that is the experimental value, what applies here is the value obtained for the given potential parameters and that may be different.
The fact that a Ca2+ cation keeps Cl- ions close is to be expected given the 2+ charge.