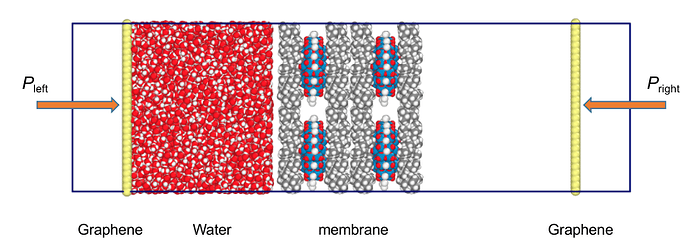
Hi,
I am studying water and ion permeation through a polyoxometalate membrane. The model uses a graphene wall as a piston to push the water through the polyoxometalate membrane. The charge on the piston wall is set to zero to avoid electrostatic interactions, and the Vander Waals interactions are modeled using LJ Potential. The piston can push the water without any challenge at a very high-pressure value (around a thousand bars which is more than what people generally use). When I use pressure in hundreds of bars (what generally people are using), the LJ interactions between water and polyoxometalate are so strong that instead of pushing the water, some water molecules are attracted towards the Piston, and some are attracted towards the membrane creating a partial vacuum in between.
Any help would be much appreciated.