Dear Mr. Gissinger,
I’m having another two questions about the Arrhenius constraint in fix bond/react. I will describe my problem in as much detail as possible.
The first question is about setting the parameter n in the map file. As shown in the following figure, we need to set the parameters A, n, Ea to invoke the Arrhenius criterion.
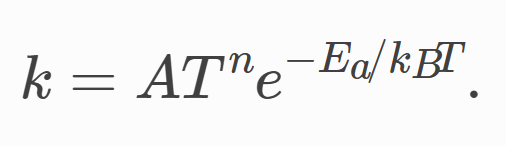
Based on previous discussions with you, I know the parameter A is to scale the Arrhenius equation between 0 and 1 for the temperature range of interest. My question is whether the setting of parameter n also serves the same purpose and does not require accurate physical meaning.
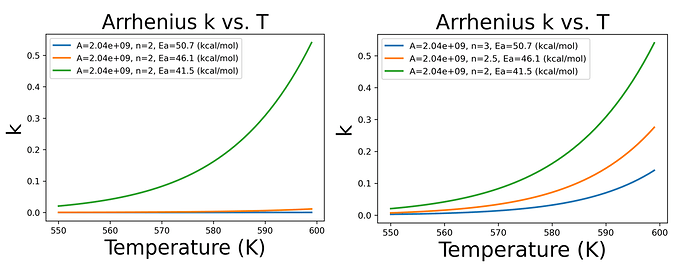
For example, as shown in the figure below, if I don’t change n, the orange and blue curves almost coincide. Only when I change the value of n can I obtain a seemingly reasonable result as shown in the figure on the right.
My second question is about the instantaneous temperature at the reaction site when each reaction occurs. After previous communication with you, I understand that the existing functions are not yet able to output the instantaneous temperature of the reaction. However, I am not sure whether the Arrhenius criterion that has been set has been triggered at a reasonable temperature.
In my computing system, I deposit multiple molecules in the box. Each small molecule has a downward velocity. If I want to calculate the overall temperature of small molecules, this temperature value will be greatly affected by this speed. Therefore, I am not sure whether the Arrhenius parameter I set can only be accurately controlled within a certain temperature range.
The above is my confusion. Thank you, Mr. Gissinger, and other friends for your valuable time and selfless help
Best wishes,
Zilin