Dear users and teachers
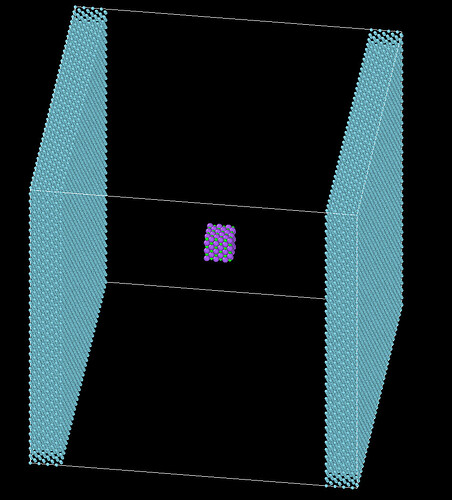
What I want to simulate is a reaction in which molten salt (1200K) drops into liquid argon (50K) at one atmospheric pressure, and there is a problem with relaxation. The molten salt will explode when I perform npt relaxation on the molten salt after relaxing the liquid argon. My guess is that there is an explosion due to the existence of a vacuum layer, hoping to find a way to relax the system here.
Attached is the systematic model, looking forward to discussing with you!
What happens when you simulate molten salt (I assume, NaCl) at 1200 K with PBC applied? This test is intended to ensure that the chosen force field correctly describes that state of matter.
It’s impossible to make any other assessment without sharing more details about your setup.
Thank you for your reply. In the system with only NaCl molten salt, I used nvt and npt successively to achieve the target from 300k to 1200k and the assumed conditions of one atmospheric pressure, and the relaxation was successful, and the density of molten salt reached the normal value. But when I used this method on molten salt in the model above, the molten salt would explode in a very short time.
You are comparing two rather different systems: in one case you have a droplet, in the other case a bulk system.
My suggestion is to forget about fix npt, but use fix nvt only and then take your combined data file and make a second copy, write two small input files where in one you delete the NaCl atoms, and in the other you delete the Argon atoms, and then try to equilibrate each system separately.
For the NaCl droplet, you may need to raise the temperature rather slowly once it is molten.
This way you can bring each subsystem to the expected starting conditions independently.
For your production run, you can then use read_data and then read_data add append to combine the two data files and then start your production simulation (which you probably should not use a thermostat at all).
Thanks for your reply, I will try the method you provided, although I don’t know whether it will be different from the experimental data.