Dear everyone,
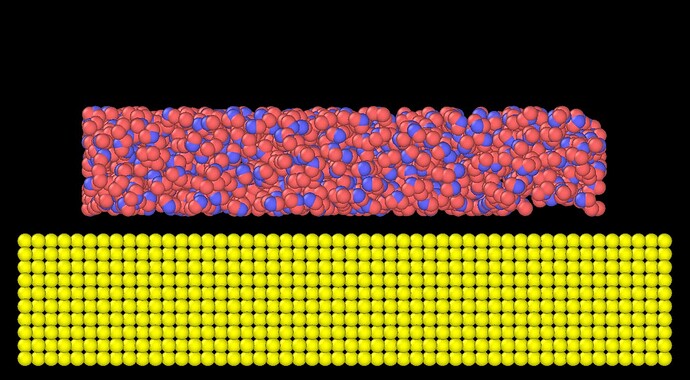
I build a water-silver interface system. 1000 H2O molecules sit above 12500Ag atoms. The initial structure looks like,
Then I used two ways of running. While holding silver atoms immobile with fix setforce 0 0 0, I used fix ‘all’ nvt and fix ‘H2O’ nvt. But I got totally different results. The key commands are the following
#----------Force field parameters----------
pair_style lj/cut/tip4p/long 2 1 1 1 0.1546 12.0 11.0
pair_modify mix arithmetic tail yes
bond_style harmonic
angle_style harmonic
kspace_style pppm/tip4p 1.0e-4
neighbor 2.0 bin
neigh_modify every 1 delay 1 check yes
# TIP4P-2005 H2O H=1,O=2
# https://doi.org/10.1063/1.2121687
pair_coeff 1 1 0.0 0.0 #H-H
pair_coeff 2 2 0.1852 3.1589 #O-O
bond_coeff 1 0 0.9572 #H-O
angle_coeff 1 0 104.52 #H-O-H
# L-J potential for Ag
# https://doi.org/10.1088/0953-8984/8/45/011
pair_coeff 3 3 7.9559 2.644 #Ag-Ag
#----------Group----------
group 1 type 1 2 #H2O
group 2 type 3 #Ag
#----------Run----------
velocity 2 set 0 0 0
fix 0 2 setforce 0 0 0
The only difference is which group to be applied for NVT.
When applying to all, it is like
fix 1 all nve
fix 2 all temp/csvr ${T} ${T} ${Tdampc} ${ransed}
When applying only to water, it is like
fix 1 1 nve
fix 2 1 temp/csvr ${T} ${T} ${Tdampc} ${ransed}
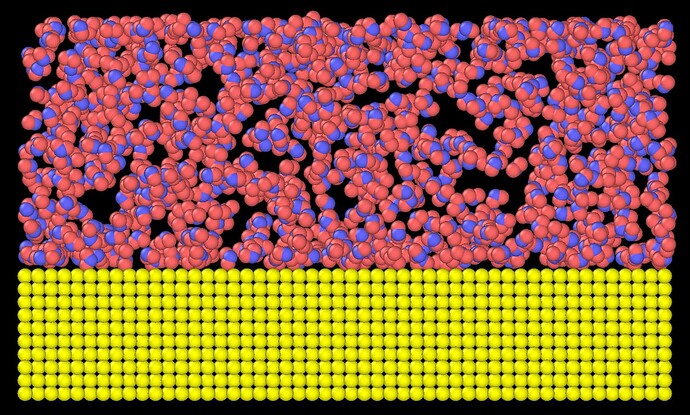
I assumed the reustls should be same since all silver atoms are not moving so that water should move the exacly same way. However, it’s not. After 200ps, the result of fix all nvt is like,
And I cant understand why water is almost evenly distributed vertically.
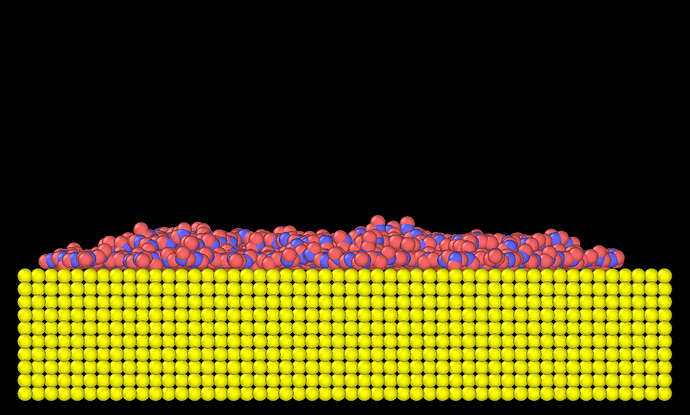
However, if it is run by fix water nvt, it is like,
Now silver is nearly hydrophilic.
What I dont understand is that why there is such a difference in water behavior when applying thermostat to all atoms or only water atoms in the situation that silver atoms are immobile by setfore 0 0 0. The interatomic force should be exactly the same but it is not.
Please enlighten me.
Thanks.